Quantum Chemistry
Unveiling Atomic Interactions with Quantum Chemical Methods
Unlocking Molecular Behavior with Quantum Chemistry
Quantum chemistry is a branch of chemistry that uses quantum mechanics to understand the behavior and interactions of atoms and molecules. It involves solving the Schrödinger equation to predict molecular properties, chemical reactions, and the electronic structure of matter.
In science, quantum chemistry provides valuable insights into material properties, reaction mechanisms, and spectroscopic data. In medicine, it aids in drug design, understanding biomolecular interactions, and studying enzyme mechanisms. In pharmaceuticals, quantum chemistry supports drug discovery, formulation development, and nanoparticle design. By offering a detailed understanding of molecular behavior, quantum chemistry plays a crucial role in advancing research and innovation across these fields.
Prediction of Binding Free Energies in Host–Guest Systems Using Extended Tight Binding Semiempirical Methods
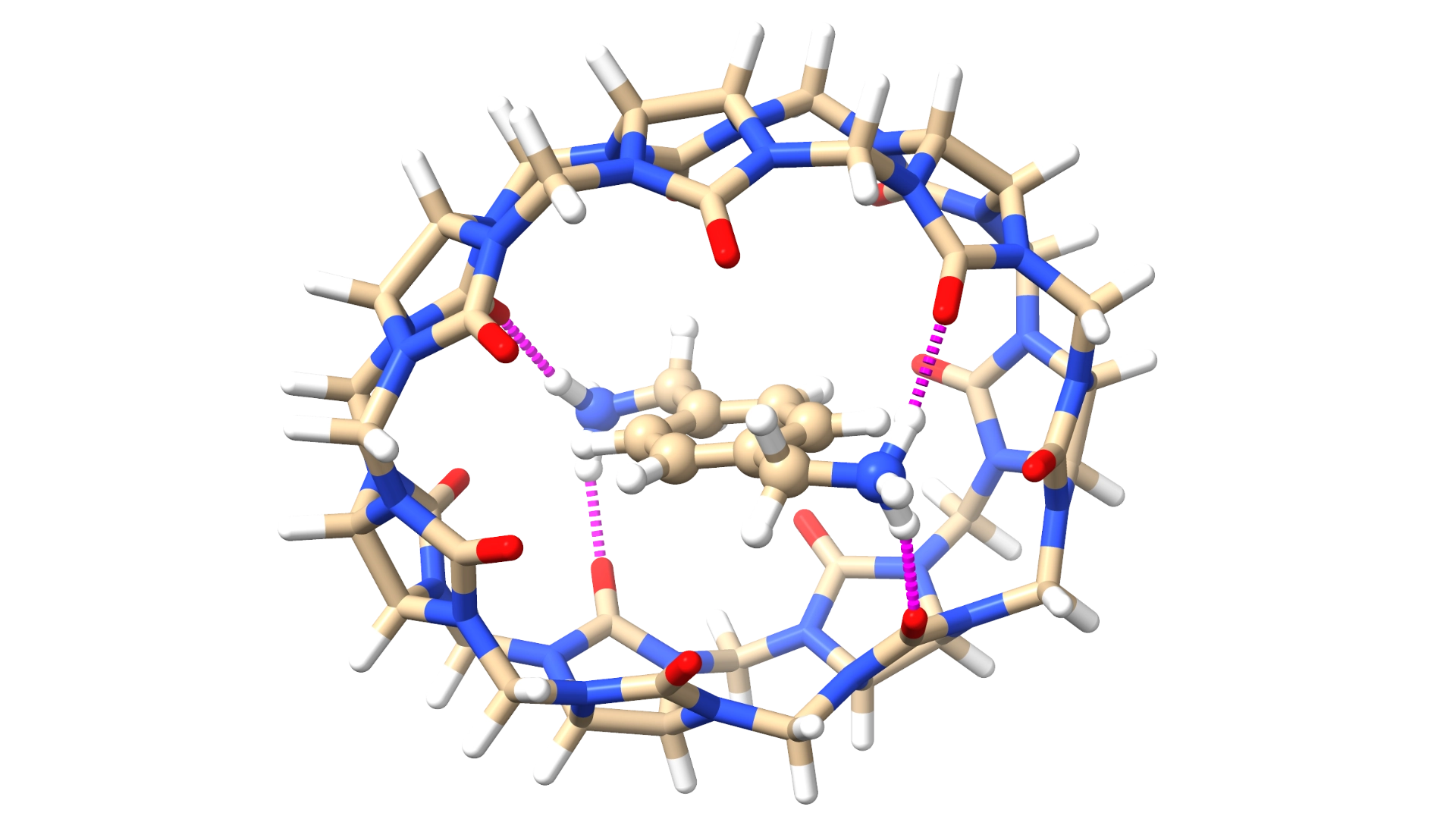
Predictions of binding energies are important for understanding how molecules interact. This is especially helpful in drug discovery, enzyme research, and materials science. These predictions allow researchers to estimate how strongly and specifically a drug will bind to its target without needing many experiments, which saves time and resources. By identifying the best candidates that bind most effectively, these predictions improve the design of drugs, biochemical studies, and new materials.
Extended Tight Binding (xTB) methods are computational tools that simplify quantum mechanics to help simulate large molecules quickly and accurately. These methods are useful for studying molecular properties and structures, providing a good balance between accuracy and computational cost, which is valuable in both materials science and biochemistry.
This research focuses on creating a reliable framework for predicting binding energies using xTB methods, particularly for host–guest systems. The study explores how cucurbiturils, which can be used as drug carriers, interact with two types of small molecules: polar or cationic drugs and hydrophobic drugs. This research aims to understand these interactions better and improve the design of cucurbituril-based drug delivery systems.
Modeling of Excited State Properties in Iron-Based Dye-Sensitized Solar Cells
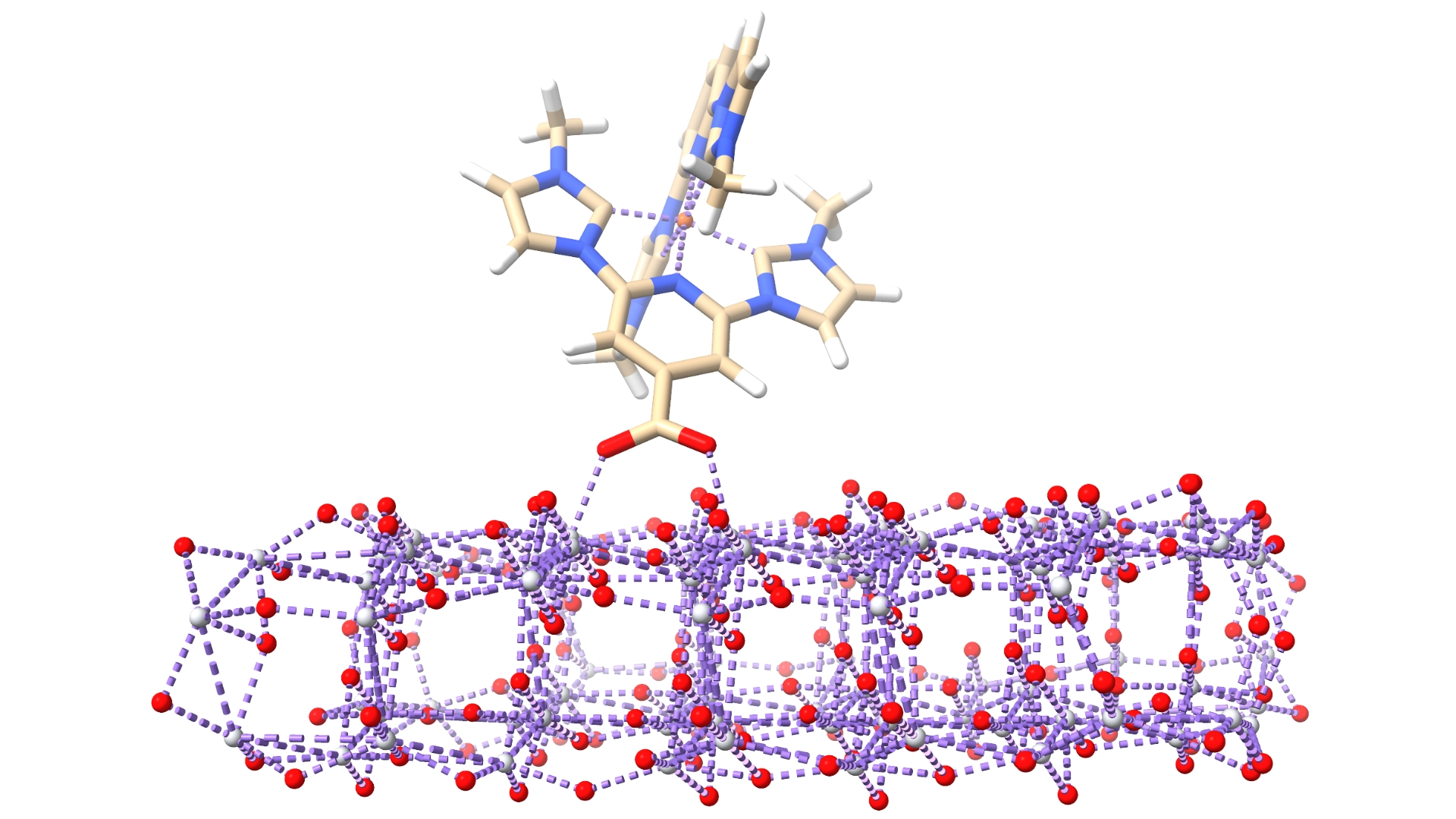
Ruthenium-based polypyridine complexes are excellent photosensitizers due to their strong light absorption and efficient electron transfer, making them highly effective in solar energy applications with conversion efficiencies up to 11%. However, ruthenium is expensive, toxic, and scarce, making it impractical for large-scale use. As a result, researchers are looking for more affordable, abundant, and environmentally friendly alternatives, such as iron.
Traditional iron(II) polypyridyl complexes show strong absorption but suffer from fast deactivation of their excited states, which prevents efficient electron injection into solar cell materials. This quick loss of energy limits their ability to convert light into usable energy. The lifetime of these excited states depends on factors like the strength of the ligands surrounding the iron and the structure of the ligands, which affect the energy of the excited states.
This project focuses on predicting how different molecular setups of iron polypyridyl complexes behave in terms of excited-state energies, how long they last, and how they interact with semiconductor materials like TiO₂. The goal is to better understand and improve the efficiency of dye-sensitized solar cells by optimizing these excited-state properties.
